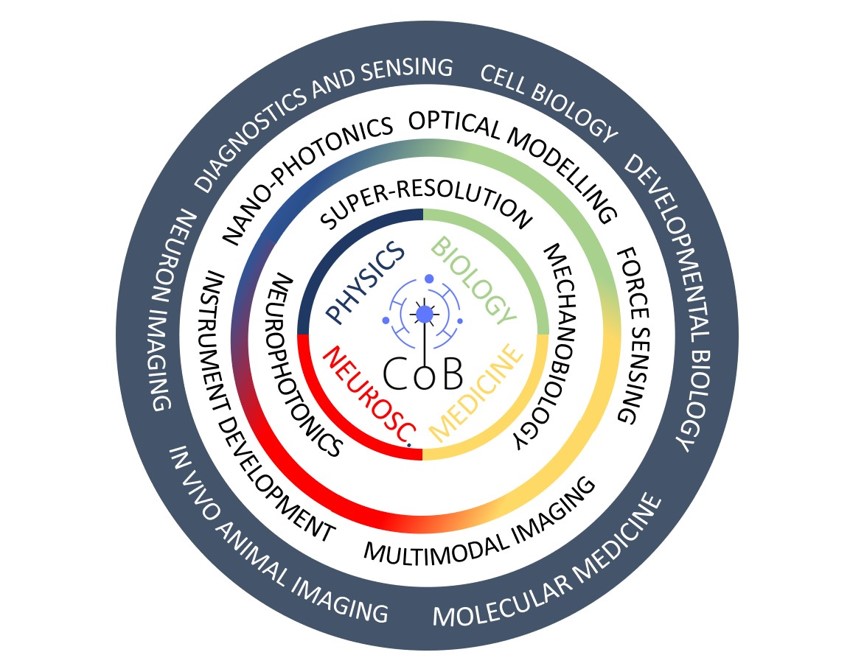
The Centre of Biophotonics (CoB) at the University of St Andrews was established in 2019 with the mission of promoting interdisciplinary research and training at the interface between advanced optical imaging, photonics and biomedical sciences. The Centre integrates researchers across four schools (Physics and Astronomy, Medicine, Biology and Psychology and Neuroscience) and builds on existing strengths in the development and application of light based technologies to investigate biological process at molecular, cellular and tissue scales. The CoB brings together more than 20 research groups around three main themes: imaging across temporal and spatial scales, mechanobiology and neurophotonics. Thus, CoB addresses important questions to improve human health including the origins of cardiovascular diseases, cancer, neurological disorders and the advance in the fight against bacterial and viral pathogens. The CoB is also strongly committed to translational research and the dissemination of technologies emerging from the Centre in collaboration with other institutions and industrial partners.

The Centre of Biophotonics is pleased to announce the 1st St Andrews Summer School in Biophotonics running from the 24th to the 28th June 2024. The Summer School is organized as a hands-on, project oriented training exercise for new as well as advanced microscopy users. Introductory classes from 9.00 to 11.00 will be followed by intensive experimental modules for small groups (4-5 people). Each module will be run by leading experts in a relax atmosphere with opportunities for networking. The school welcomes postgraduate students, post-doctoral researchers and early career group leaders across the physics and life sciences with an interest in cutting-edge microscopy.
More information regarding specific modules, registration and accomodation at discounted rate at St Andrews University residences can be found here.
Seeing is believing and light-based imaging technologies are, now more than ever, uniquely positioned to unveil the mechanisms of life as well as disease. Building on more than 20 years of light-based innovation for the biosciences and by collaborating across disciplines and recruiting the best talents, we aim to watch these processes unfolding in real time, from the molecular and cellular scales, to the whole-organism level.









Recent publications
Participating Schools
Latest News
- 1st Joint Biophotonics Webinar Gruss Lipper Biophotonics Centre (Albert Einstein College of Medicine) and the Centre of Biophotonics-CoB (St Andrews)Promoting Light4Life alliances across the atlantic 15th March 2024 14.00-17.00 UK Time https://einsteinmed.zoom.us/j/93406082376
- Lights, lasers and pizza!: Equate MeetUp with Opening Up PhotonicsThis event is open to all women & non-binary students in STEM subjects at Scottish Universities and Colleges. Light and Lasers: Equate MeetUp with Opening Up Photonics Tickets, Mon 20 Nov 2023 at 17:30 | Eventbrite Equate is delighted to be partnering with Opening up Photonics and the St Andrews’ Centre for Biophotonics for our…
- Open: Expressions of interest for PDRA fellowship applications at the CoBThe CoB is currently open to expressions of interest for researchers interested in applying for postdoctoral positions hosted at the Centre of Biophotonics under the Marie Curie PDRA scheme and the Royal Society Newton International Fellowship Scheme. Contact us as quickly as possible, more information can be found here
Next BioLIGHT CoB Seminar:
Imaging Protein-Nucleic Acid Interactions Across Scales and with Single Molecule Resolution

Speaker: Prof David Rueda, Imperial College London.
Faculty of Medicine, Department of Infectious Disease
Chair in Molecular and Cellular Biophysics
Host: Prof Carlos Penedo
1st May 2024, 1 pm, BMS Seminar Room
Over the past decade, the market in RNA-based therapeutics and technology has emerged rapidly in the field of life sciences, experiencing a sizeable compound annual growth rate. Examples of early FDA-approved RNA-based drugs are spinraza, an antisense oligonucleotide developed to treat spinal muscular atrophy by regulating mRNA splicing, and patisiran, based on RNA interference to treat hereditary transthyretin-mediated amyloidosis. Other key RNA-based technologies are CRISPR/Cas9 for gene editing and, most recently, the latest RNA vaccines by Pfizer and Moderna to treat COVID-19.
To understand how RNA therapeutics function and to further develop new RNA-based drugs, it is essential to elucidate their mechanism at the molecular level. Single-molecule microscopy approaches provide unique opportunities to investigate fundamental biological processes involving nucleic acids and proteins. By eliminating ensemble-averaging, they enable monitoring such processes in real-time, providing an opportunity to track dynamic events, molecular interactions, and the formation of large macromolecular complexes and transient biomolecular species with unprecedented resolution.
Our laboratory develops and applies fluorescence- and force-based single-molecule microscopy approaches to study fundamental biological processes involving RNA molecules and RNA-protein complexes. In this presentation, we will present our most recent efforts to determine the molecular factors that lead to CRISPR/Cas9 off-target activity and our recent advances using fluorogenic RNA aptamers for background-free imaging of cellular RNAs with single-molecule resolution.
His full profile can be found here:
https://www.imperial.ac.uk/people/david.rueda