Facilities
We have multiple light microscopes and workstations dedicated to image processing. Some of our microscopes are commercial instruments but a significant number of them are innovative techniques developed by groups within the Centre, and therefore, are custom-built setups. Our imaging facilities are additionally complemented by nano- and microfabrication techniques within a cleanroom environment and ultra-fast techniques including femtosecond spectroscopy, fluorescence up-conversion and transient-absorption spectroscopy. This provides a broad experimental platform to address important biological questions across temporal and spatial scales and drive innovation. Below, we list some of our instruments and facilities. If you are interested in having more information as a potential user please contact us.
Equipment – Commercial light microscopes
ZEISS AiryScan

Location: BSRC Level 1, School of Biology, Contact: Dr Marcus Bischoff
LEICA TCS SP8

Location: Room B122, Biomedical Science Research Complex (BSRC), School of Biology.
ZEISS Axioplan 2

Location: Room B122, Biomedical Science Research Complex (BSRC), School of Biology.
ZEISS Pascal 510 confocal
Location: Room B106, Biomedical Science Research Complex (BSRC), School of Biology.
ZEISS Apotome

Location: School of Psychology and Neuroscience
Crest X-light confocal

Location: School of Psychology and Neuroscience
Spinning disk confocal

Location: Wolfson laboratories. School of Medicine.
NIKON N-SIM

Location: School of Physics and Astronomy
ZEISS Axioscan Z1
Location: School of Medicine
Deltavision

Location: ROOM B104, Biomedical Science Research Complex (BSRC), School of Biology.
ZEISS Axiovert – Microinjection
Location: School of Physics and Astronomy
LEICA LMD6500

Location: School of Biology, BSRC Annex
Equipment – Custom-built microscopes
Total-Internal Reflection (TIR)
Location: Prism-type TIR Level 1 , Biomedical Science Research Complex (BSRC), School of Biology. Objective-type TIR:
Single-molecule FRET
Location: Level 1, Biomedical Science Research Complex (BSRC), School of Biology.
Single-molecule spectrograph
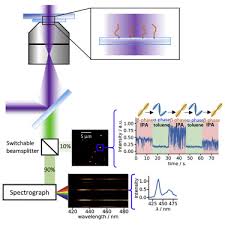
Location: Room 268, School of Physics and Astronomy
Single-molecule FCS-MFD
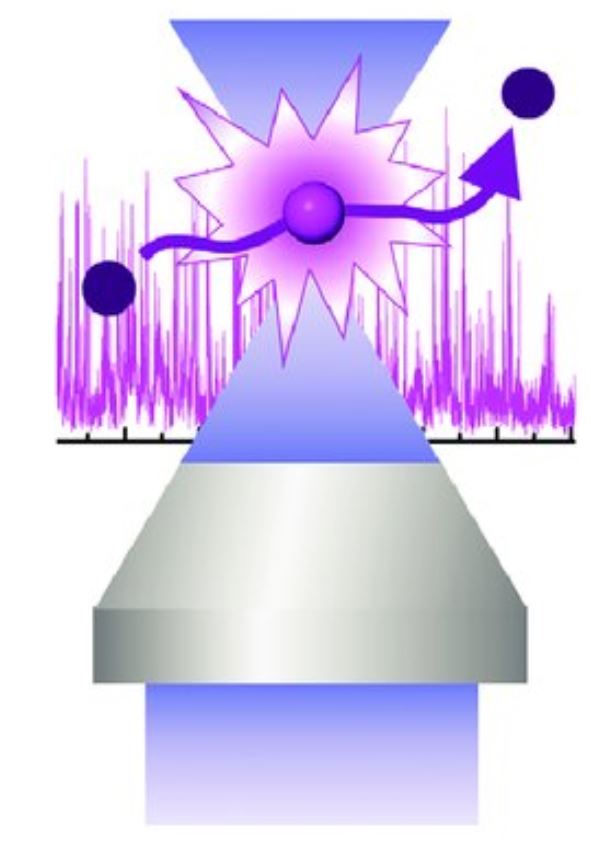
Location: Biomedical Science Research Complex (BSRC), School of Biology.
Combined magnetic tweezers and fluorescence imaging
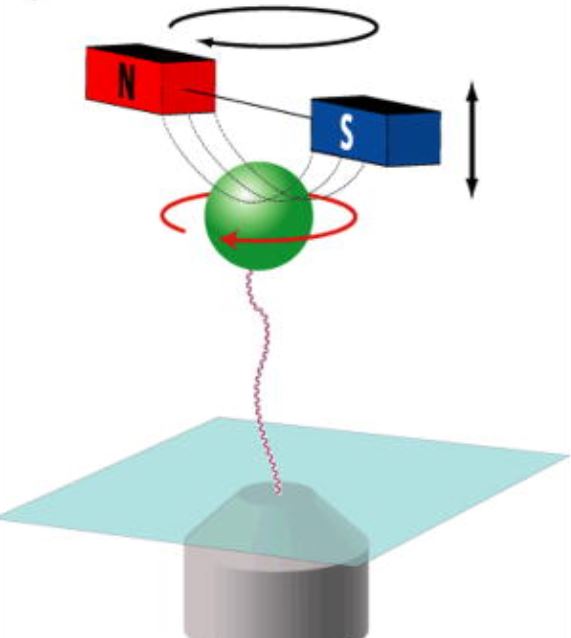
Location: Rom 268, School of Physics and Astronomy
TRAFIX multiphoton microscope
TRAFIX: temporal focussing with single pixel detection for imaging at depth.
Location: School of Physics and Astronomy
Custom light sheet microscopes
Location: School of Physics and Astronomy
Raman/holographic microscopy
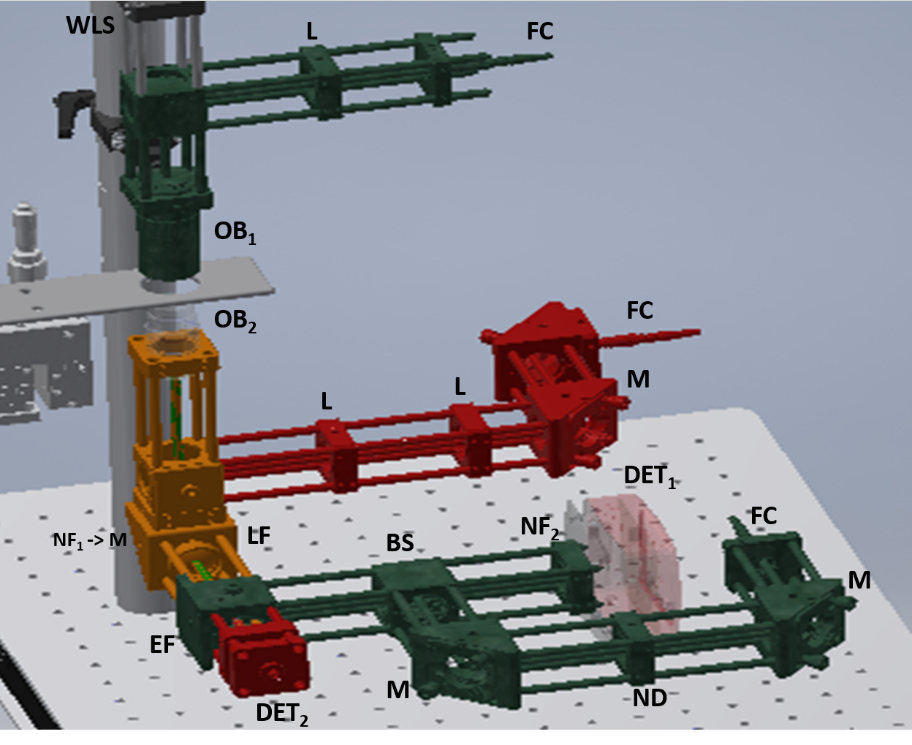
Location: School of Physics and Astronomy
Acoustic trapping/Raman spectroscopy platform
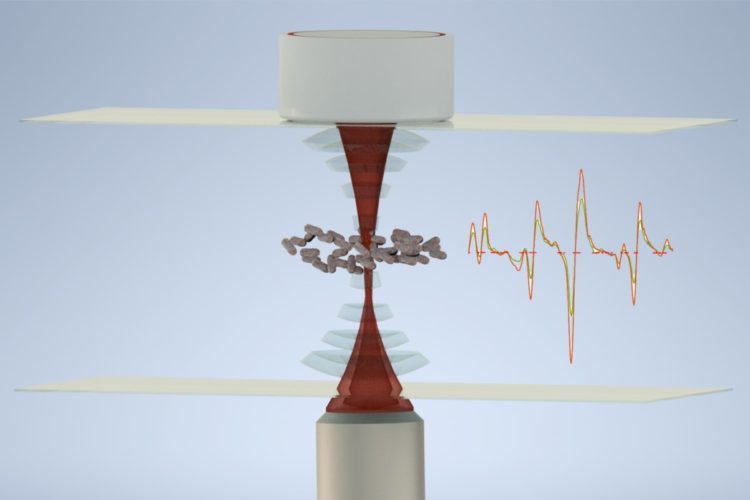
Location: School of Physics and Astronomy
Femtosecond spectroscopy facilities

We have access to a number of instruments for femtosecond spectroscopy including:
- Femtosecond lasers (SpectraPhysics Mai Tai Ti:Sapphire oscillator – 80 MHz output, 750-850 nm, 100 fs FWHM pulse width, SpectraPhysics Merlin laser at 527 nm, 50 KHz and 200 ns pulse width)
- Light amplifiers (SpectraPhysics Hurricane, SpectraPhysics OPA, Light-Conversion TOPAS-White and SpectraPhysics Spitfire.
- Hamamatsu Streak Camera – Detection of luminescence from thin films or solutions, with an instrument response function of ~ 2 ps (FWHM).
- CDP FOG 100 – Optical gating allows luminescence to be detected on the femtosecond timescale. By sum-frequency mixing of the gating pulse and sample luminescence, upconverted light is produced that is detected. This technique allows temporal luminescence kintetics with time constants as low as 50 fs to be resolved.
- Homebuilt Transient Absorption – Utilising two beams, a pump and a probe, with one of them delayable, allows transient kinetics in the absorption of samples to be recorded on the femtosecond timescale.
- These facilities are hosted at the School of Physics and Astronomy and coordinated by the labs of Prof . Samuel and Prof. Turnbull. (Image courtesy of Ifor Samuel/Graham Turnbull)
Nanofabrication facilities

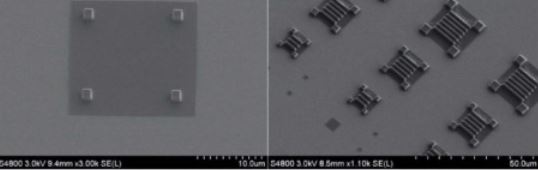
We have facilities to manufacture micro- and nano-patterned photonics structures via electron-beam lithography and to characterize them via AFM, 3D microprinting, DEKTAK profiler and EM. If necessary, the fabrication process can be carried out in a cleanroom facility and entirely in a nitrogen atmosphere glovebox. These facilities are hosted in the School of Physics and Astronomy and managed by Prof Samuel, Prof Turnbull, Prof. Malte Gather and Prof di Falco. In addition, instrument developers at the CoB have access to excellent electronic and mechanical workshops to assist the researchers in the design, drawing and manufacturing process of specialized components. (Image courtesy Andrea di Falco).
